Light Sheet Microscopy (LSM) has re-emerged as a technique to provide detailed microscopy images from large, uncut samples with a resolution comparable to that of confocal microscopy. The ability to image samples without intensive fixation, embedding or slicing procedures is of special interest for physiological studies, where sample integrity is critical to obtain relevant results. We have extended this concept to the imaging of live plant samples in culture, registering gene expression events in A. thaliana roots while they grow under sterile conditions. Although gravitropism determines that roots grow downwards, interaction with the medium provides tactile clues that result in the root tip following a three-dimensional path. Growing the plants on vertical agar plates simplifies imaging to 2D, but doesn´t correctly replicate root-medium interaction, as it is then asymmetrical. On the other hand, growing the seedlings on horizontal plates leads to roots entering the medium and becoming difficult to observe. Taking advantage of the unique design, high speed and sample-handling flexibility of QLS-Scope, we have devised a culture set up that allows an easy, fast and sensitive approach to molecular plant physiology at 5D level or even plant-plant interaction studies

Figure 1. Arabidopsis plant grown in the observation tube. The optical features of FEP allow photosynthesis and growth for at least 7 days while providing a matched RI.
| Figure 1. Arabidopsis plant grown in the observation tube. The optical features of FEP allow photosynthesis and growth for at least 7 days while providing a matched RI. |
MATERIALS AND METHODS
Solid medium (0.4% agarose, 1% sucrose, 0.5xMS pH=5.8) was autoclaved and 200 µL were dispensed into sterile 4 mm Ø FEP (Fluorinated Ethylene Propylene) tubes (Norell) sealed at the bottom with Brand™ sealing wax. Seeds from the following SAND lines[1] were sterilised using a standard bleach/ethanol/sterile water wash protocol and sown inside the tubes:
- SAND2106110: GFP expression under the atHB8 (at4g32880) promoter
- SAND2106120: GFP expression under the IRT1 (at4g19690) promoter
Each tube was closed with sterile cotton and placed vertically in a closed, transparent container to limit evaporation. The container was placed in a growth room under a 16/8 hour light/darkness cycle at 25ºC. After 4 days, seedlings had germinated and roots of about 1 cm long were ready for imaging (Figure 1). For additional tissue definition, we opened the tubes under sterile conditions and added 50 µL of 25µg/ml propidium iodide (Sigma) diluted in water. After allowing diffusion for 30 minutes, the tubes were directly introduced in the QLS-Scope sample holder and automatically placed into a cuvette with a liquid of matching refractive index (1.34). The plants were observed with laser/filter combinations of 488 nm/ 531(40) BP (GFP observation) and 515 nm/655(40) BP (propidium iodide observation). Images were taken with a 10x/NA 0.3 water immersion objective. The resulting files were directly open and processed using FIJI[2].
[1] Plant J. 2016 January; 85(2): 320–333. doi:10.1111/tpj.13099.
[2] Schindelin, J.; Arganda-Carreras, I. & Frise, E. et al. (2012), “Fiji: an open-source platform for biological-image analysis”, Nature methods 9(7): 676-682.
RESULTS
PLANT GROWTH IN THE IMAGING SET UP
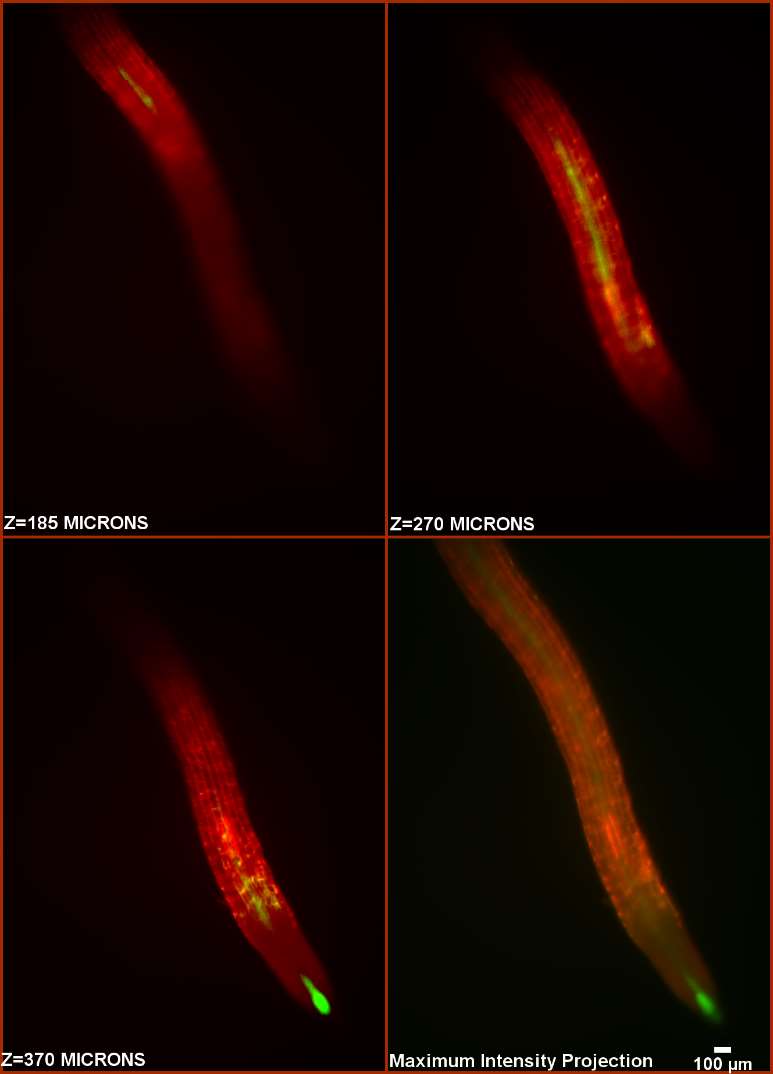
| Figure 2. Captures of a SPIM series (150 sections at 4 micron steps) through a growing atHB8p:GFP root. Captions in the first 3 frames indicate depth along Z. The 4th frame shows a projection of the complete series. A high definition image series is available upon request. |
Seed germination and growth inside the imaging tubes was optimal (100% germination rate, n=20), and plantlets penetrated the agarose medium without obvious problems. In general, after 4 days all plants presented a primary root of approximately 1 cm in length. The tubes allowed proper illumination for plant growth, and using an external container permitted adequate respiration without excessive evaporation or medium desiccation.
3D-TRACKING OF ROOT GROWTH
The use of light sheet microscopy allows live visualization of the root without disturbing its growth, breaking sterility or interrupting the experiment. We have tested this concept by developing an experimental setup that makes use of the high resolution, optimized optical features, high speed and simplicity of use of QLS-Scope to follow root growth along its 3D pathway. Figure 2 shows a root expressing atHB8-driven GFP at the growth tip and along the stele. The measurements were taken in a single SPIM comprising a volume of 2.2*3.1*0.6 mm (4.1 mm3), which took only 45 second to complete, thus reducing the time the plant was under analysis and potential experimental alterations. Furthermore, the addition of diluted stain to the growth medium did not result in either increased background or obvious growth alterations.
IMAGING THE INTERACTION SPACE
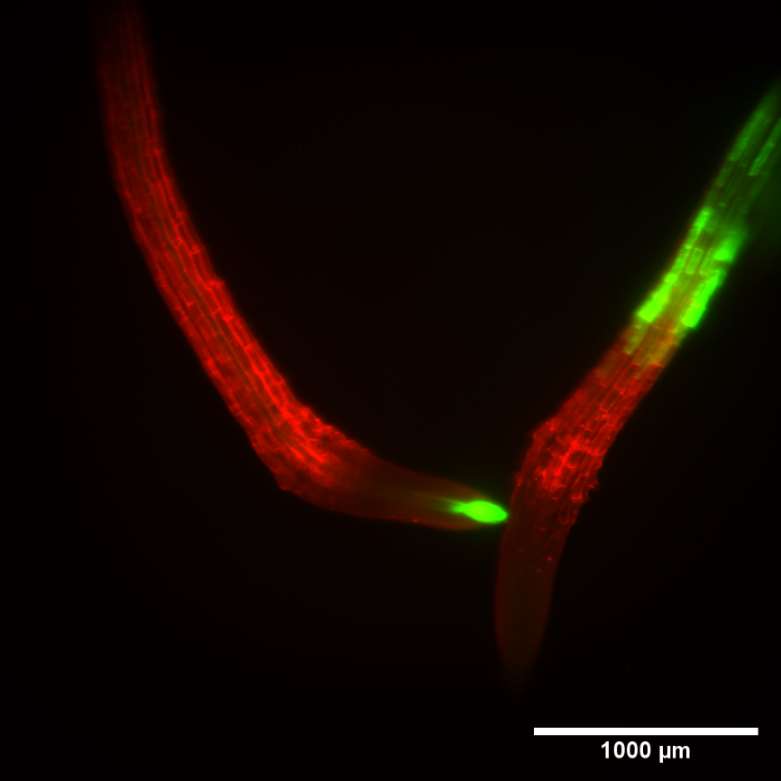
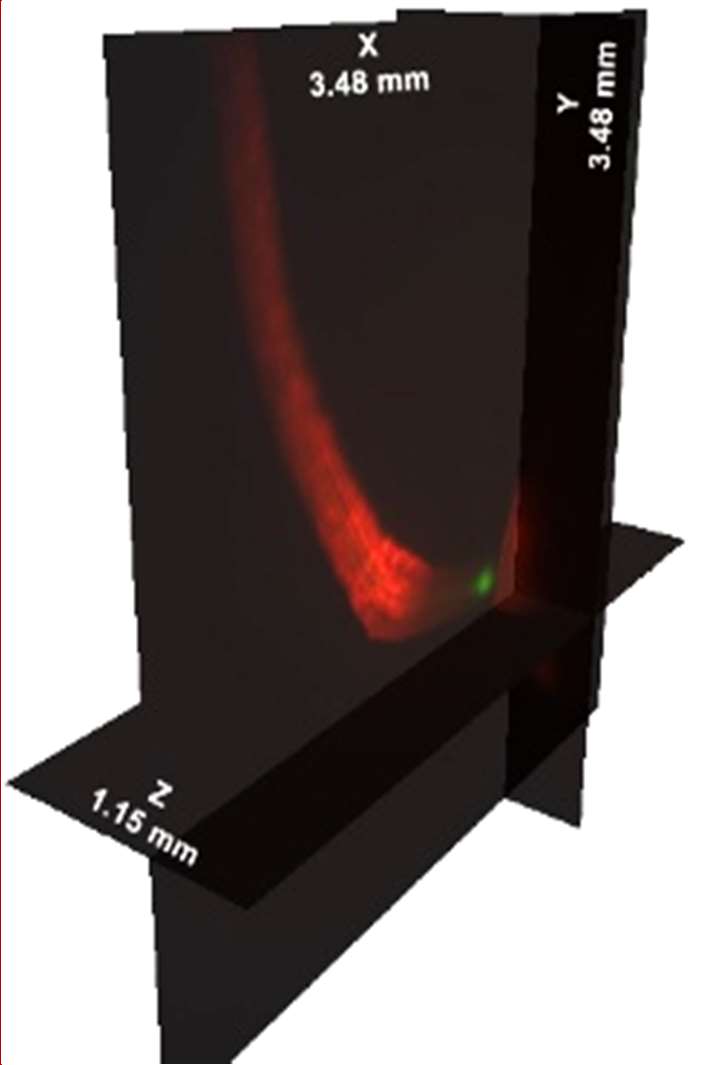
| Figure 3. SPIM measurement of 2 roots from different genotypes developing in the same culture tube. Left panel, maximum intensity projection of 340 sections at 3.4 micron steps. QLS-Scope can image both individuals simultaneously, clearly showing individual cells and the corresponding promoter-driven expression patterns. Right panel, orthogonal projection of the series, displaying the dimensional depth of the experimental volume. A high definition image series is available upon request. |
The dimensions of the imaging/culture tube (4 mm internal diameter) allow plant growth for at least 7 days, as well as the design of live interaction studies. To test the concept, we co-cultured 2 different genotypes (atHB8p:GFP and IRT1p:GFP) and allowed the seedlings to grow for 4 days before staining and imaging them. Figure 3 shows the root tips photographed with SPIM at 10x magnification. The image series covers a volume of 14 mm3, and was taken in 50 seconds. The ability to image a large volume within the culture system may be of use in multifactorial studies, such as fungal infection dynamics, establishment of rhizobial symbiotic processes, ethylene-based plant-to-plant communication or direct comparison of mutants under hormonal treatments.
CONCLUSION
QLS-Scope presents a number of features (high scanning and acquisition speed, sample size flexibility, adaptability to different RIs) that expand the possibilities of microscopy-based research. Here we have described an application to Arabidopsis thaliana studies during in vitro culture that offers the chance to perform high quality imaging without breaking sterility or even disturbing plant growth conditions for more than a few minutes. We believe QLS-Scope is a valuable tool for plant biology and will expand the depth of genetic and physiological research in the field.
ACKNOWLEDGEMENTS
We wish to thank Professor Gregorio Hueros at the University of Alcalá, Madrid (Spain) for his collaboration in establishing the imaging/culture set-up.
Application Note
Luis Muñiz, PhD
Nikos Ekizoglou, PhD
Prof. Jorge Ripoll
PlaneLight SL
November 2019